Description
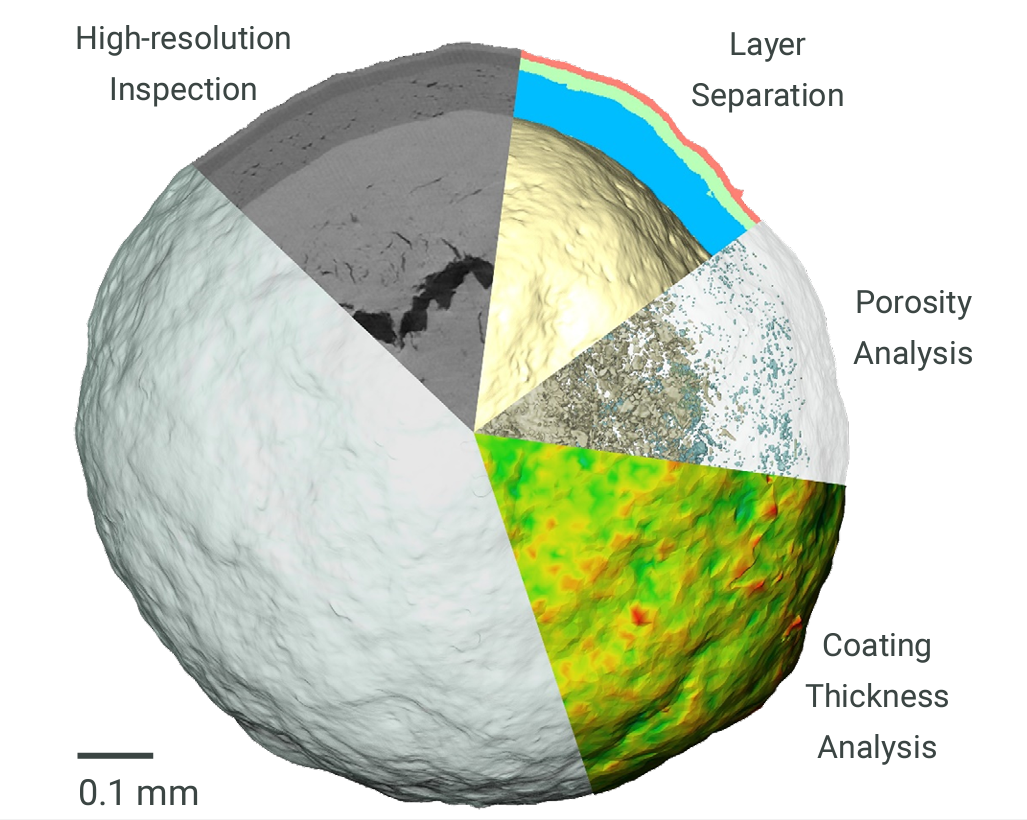
The microstructure of solid dosage forms of pharmaceutical products is a critical factor that impacts disintegration and dissolution rates. As such, the microstructure will also play a key role in bioequivalence and therapeutic equivalence. Being able to image the microstructure of a solid dosage form allows optimization of production and formulation procedures to achieve a robust dissolution response. If an out-of-specification dissolution result is later observed, analysis of the solid dosage form’s internal structure and microstructure can yield a wealth of insights not accessible through traditional analytical approaches and help resolve mission-critical investigations. New product development can be a highly time-consuming and expensive task. Using Rigaku’s nano3DX, this process can be accelerated, providing immediate feedback on a product’s internal structure when any discrepancies between expected and actual attributes can be identified.


 Share
Share
