12. Dec. 2024
Scientists at CEITEC Masaryk University (MUNI) have made a discovery that could significantly impact treatments for diseases associated with harmful amyloid-like protein aggregates. Their study focuses on how cells maintain a dynamic balance between different protein states so that proteins can function properly. This discovery paves the way for the development of new therapeutic approaches for diseases associated with protein disorders.
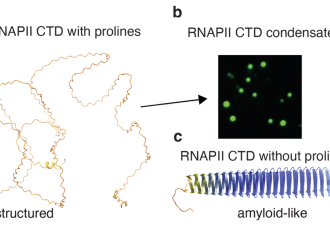
Proteins, crucial molecules for all life functions, adopt various physical states within cells, ranging from liquid to solid forms, to perform their roles properly. They also often exist in intermediate states distinguished by a process called phase separation, where they aggregate into droplet-like structures known as condensates. These condensates allow proteins to interact dynamically and efficiently, which is essential for the proper functioning of cells. However, when this delicate balance is disturbed, the proteins can 'solidify' into amyloid-like structures which are linked with neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and Alzheimer's disease.
In their study published in the prestigious journal Nature Communications, the researchers focused on the enzyme RNA polymerase II, which plays a crucial role in reading and transcribing genetic information in our cells. The research team, led by Richard Štefl and Robert Vácha from CEITEC MUNI, discovered through a combination of experimental techniques and computational modelling that this enzyme contains a region rich in the amino acid proline, which is key to forming droplet-like condensates and prevents RNA polymerase II from solidifying into harmful aggregates. This enzyme’s ability to form condensates is crucial, as it enables it to efficiently conduct gene transcription.
However, the study revealed that phosphorylation, the process by which an enzyme adds a small chemical group (phosphate) to a protein substrate to regulate its function, has a significant effect on the transition of RNA polymerase II between material states. "We have observed that when RNA polymerase II and other molecules cluster into small droplet-like structures, the whole phosphorylation process is accelerated several-fold. This means that these droplets accelerate important biochemical reactions in the cell that the enzyme needs to function properly," says Kateřina Linhartová, lead author of the study. "This discovery may help scientists understand why harmful protein solidification occurs that has been linked to neurodegenerative diseases. If scientists can learn to control this process, they could develop new therapeutic approaches to prevent or even reverse the progression of these diseases," adds Richard Štefl, leader of the research team.
Biomolecular condensates, once a relatively overlooked phenomenon, are now gaining the attention of the pharmaceutical industry. As scientists delve deeper into the impact of phase separation on cellular functions, the potential for applying these findings in enzyme engineering and drug development is expanding.


 Share
Share