15. Aug. 2024
Bacteriophages are viruses that multiply in bacterial cells. When a bacterium releases phage progeny, the bacterial cell bursts and dies. Because of this effect, phages are beginning to receive more attention as potential treatments for antibiotic-resistant pathogenic bacteria. Pavel Plevka's research team at CEITEC Masaryk University has now described the structure and replication cycle of the bacteriophage JBD30, which infects and kills Pseudomonas aeruginosa. This bacterium causes life-threatening infections in people with weakened immune systems.
Although phages are the most abundant biological entities on the planet, many aspects of their life cycle remain unknown to us. The phage JBD30, studied by structural virologists at CEITEC Masaryk University, infects P. aeruginosa. This human pathogen causes respiratory and urinary tract infections and severe infections in people with weakened immunity or chronic diseases such as cystic fibrosis. Structural virologists used cryo-electron and super-resolution fluorescence microscopy to describe how phage JBD30 infects P. aeruginosa.
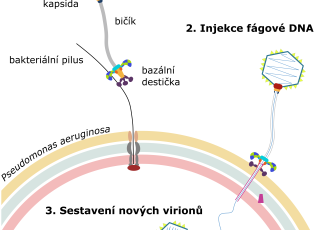
The particle of phage JBD30 is formed by a head and a tail, shaped like a long tube. Like most viruses, phage JBD30 is very small, with a particle length of 0.0002 millimeters, which is approximately ten times less than the thickness of a spider web filament. The end of the JBD30 tail is equipped with hooks that enable the phage to attach to bacterial pili. The pili are thin filaments that the bacteria repeatedly extend and retract, and, therefore, they can serve as throwing anchors that the bacteria use to move over surfaces. "The bacteria use pili to move, explore their surroundings, and form biofilms. The alternating extensions and retractions of pili are essential for bacterial movement, which JBD30 cleverly exploits. Using the hooks at the end of its tail, the phage attaches to a pilus and waits to be pulled to the bacterial surface," says Lucie Valentová, the research paper's lead author. In addition, the specificity of the interaction between the phage tail hook and the pilus orients the phage particle in such a way that the attachment of the phage to the cell resembles the landing of a space module on the surface of the Moon. "Upon landing on the surface, the tripod of phage receptor-binding proteins opens and releases proteins from the end of the tail that form a channel across the bacterial cell wall. The phage then uses this channel to inject its genetic material inside the cell," adds Pavel Plevka. "The phage DNA then takes control of the bacterial cell and uses it to copy itself and assemble new phage particles," concludes Lucie Valentová.
The research results, published in the EMBO Journal, also reveal what happens in an infected bacterial cell. "We took pictures of progeny phage particles assembling inside the cells. The phage heads first assemble in an immature form and then become filled with DNA carrying the phage's genetic information. The full heads are then joined with tails that had assembled independently," explains Pavel Plevka. At the end of the infection cycle, the phage produces enzymes that degrade the bacterial cell wall from the inside until the cell bursts. "Under a microscope, the phage-induced bacterial lysis looks like fireworks. The cells burst and release new phages into their surroundings," concludes Lucie Valentová. The way phage JDB30 infects and kills bacteria differs from the mechanisms of action of antibiotics, which block specific vital functions of bacterial cells. Therefore, phage JBD30 can successfully attack even antibiotic-resistant P. aeruginosa.
These findings help scientists understand how phages recognize host bacteria and interact with them. By identifying and revealing the structures of key proteins involved in phage multiplication in the bacterial cell, scientists can contribute to the development of phage therapies. This research was supported by the National Institute of Virology and Bacteriology Exceles project.


 Share
Share