15. Aug. 2024
Scientist Zdeněk Jakub from the research group Molecular Nanostructures at Surfaces, led by Prof. Jan Čechal at CEITEC BUT, reveals in his latest publication the important role of the substrate on the properties of metal-organic frameworks (MOFs). These materials, prized for their flexibility and wide range of applications in catalysis and quantum computing, are now being studied in more detail thanks to a unique approach using graphene. The research shows how graphene minimizes the influence of the substrate, opening up new possibilities for optimizing these materials.
I understand that graphene is a very popular material now. Is that why you used it?
ZJ: We are primarily interested in the metal-organic frameworks (MOF) on the surface, and we use graphene to limit the influence of the substrate. 2D MOFs are materials in which metal atoms are bound via organic molecules into networks where these components alternate regularly. They are great materials with almost limitless modification possibilities: we can choose the metal and the molecule, allowing us to tailor their properties. If we want to study these networks with atomic resolution, we need to prepare them on some surface to do the measurements. However, a potential issue is that these surfaces are usually metals. While these are are practical and easy to work with, they change the properties of the material of interest.
Was the problem of the surface changing the properties of the MOF known?
JČ: It was known that the surface affects the properties of the network, but it was not known the extent because almost nobody can prepare these networks on graphene.
ZJ: Yes, it was known, but it was hard to quantify. It always depends on the particular property in which we are interested. For some properties, the effect of the substrate may be minimal. We did one of the first detailed studies – we compared the same MOF material on a traditional gold substrate and graphene. We studied the differences and we described how the support defines the individual properties of the 2D MOFs in the context of different applications. In particular, we have shown that, for example, the adsorption properties of our MOF are significantly affected by the metal substrate. This is important for further research, e.g., in catalysis – now it is clear that if we search for new 2D materials for catalytic applications, we cannot use metal surfaces and expect similar results in real systems. In general, our work helped to better understand how to isolate material properties from surface effects.
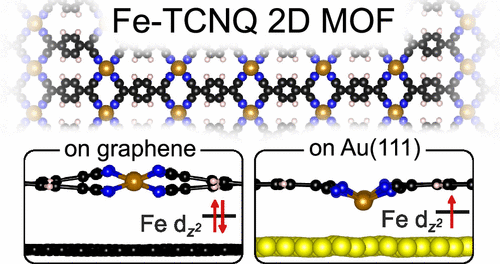
So, with this publication, are you recommending that scientists should use graphene as a substrate when working with metal-organic networks?
ZJ: Basically, yes, I think that’s a way to minimize the influence of the substrate in various applications – if that’s what’s needed.
In general, our work consists of creating so-called model systems to gain fundamental insight. Having them, we are able to estimate what parameters need to be optimized in a real system. Parameters such as the geometrical arrangement of the lattice, the electronic structure of the individual atoms, and the possibility of lattice deformation change basically everything. We can change these parameters in a controlled way and study how the material properties change. When a chemist prepares a similar material in solution, they usually do not have such precise control over the individual parameters. We are able to study these details with atomic precision. That is the main value of our work because we can then determine what material parameters need to be optimized for specific applications.
JČ: It’s like having a map – it’s easier to find a castle with it than to wander around the countryside pointlessly. To put it simply, we provide a map that makes it easier to find the optimal solution.
Graphene is a very popular material at the moment; why did you start researching it?
JC: I became interested in graphene because we can externally control its electronic properties. It is a very thin material – a single layer of carbon atoms. Using an external electric field, we can add or remove electrons to it, which can affect other materials that are in contact with it. I’m interested in externally tunable materials – imagine a material whose properties are changed by turning a control knob. For example, we use a voltage source and set it to, say 7 V, and the material starts to catalyze reactions. If we change the voltage, its catalytic properties will change, too. However, for this, we need a graphene device, a field-effect transistor, which would have a surface free of any impurities and hold up under a vacuum. We haven’t achieved that yet, but we’re on the way. So far, we’re studying very pure graphene, which we produce directly in a vacuum.
What is the biggest contribution of your publication?
ZJ: It opens the way for experimental studies of the fundamental properties of 2D metal-organic materials. There are lots of amazing predictions based on computer simulations, but experimental verification is more difficult. The predicted properties are often absent in real systems due to the influence of the substrate – what was predicted for an ideal isolated material is no longer valid for a material laid on top of a metal. We show that the predicted properties can be realized if we use a suitable substrate. Another thing that Jan mentioned is that graphene can be tuned by an external field. Even if we use the same material, the very same metal-organic network, one can change its properties by slightly charging the substrate – adding or taking away electrons. And that’s what we’re able to do with graphene. We cannot implement it in a device yet, but the potential to develop tunable materials is huge.
That could lead to what?
JČ: We could create much more efficient catalysts, for example. Let me try to explain. A catalyst needs to bind reactants and release products after the reaction is complete to open the catalyst for the next reaction. With traditional catalysts, you have to find a compromise – they cannot be too excellent at one thing without adversely affecting the other. Dynamic materials that can be quickly switched between different states can solve this problem. Then, the catalyst has to be optimized for each process separately – for reactant binding, reaction, and product release. Then, cyclic switching can achieve multiple efficiencies.
What does the material preparation look like? What equipment do you work with?
ZJ: We do everything in ultra-high vacuum to achieve atomic precision. We use very pure, polished single crystals of metal. We can see the atomic steps and the individual atoms on the surface. Then, when we deposit organic molecules and metal atoms on that surface, we can see how they are arranged, and we can measure their structure with scanning tunneling microscopy, electron diffraction, and photoelectron spectroscopy.
Then, we combine experimental studies with theory, which is very important. Much of the work is done by our colleague Jakub Planer, a computational physicist. He tests various computer models and compares them with experimental results. This is another great value of our work – our systems are well-described both experimentally and theoretically, which is essential for a detailed understanding of the properties of materials and also the development of new computational methods.
What else does your research group focus on besides metal-organic networks?
JČ: Apart from catalysis, we also focus on magnetic properties towards quantum applications. One of our projects focuses on the surface of a topological insulator with robust surface currents. If we modify the material, we can create one-dimensional spin conductors. These conductors are crucial for quantum computers because they allow the lossless transfer of spin information between quantum bits.
What applications would you like your materials to be used in?
JČ: I would like to see that our material systems are a key part of the next generation of quantum computers. Our materials would serve as both qubits and quantum conductors. Also, I would also like to develop much more efficient catalysts and electronics.
ZJ: I think it would be great if we could create catalysts with exact specifications. For example, if we want a certain reaction to proceed selectively, we could first figure out what atoms and geometric and electronic configurations are needed and then build the resulting material exactly that way.
JČ: We try to understand the materials first and foremost. If we understand them, we can say, yes, we need to do this, that, and the other to make the material have the properties we want. So, we strive to understand them theoretically and experimentally. It’s important to know what is going on. To say that it is better, but also why it is better.


 Share
Share




