1. July 2019
 EU-LIFE, the alliance of 13 leading life science institutes in Europe, points out the increasing lack of opportunities for collaborative research in biomedicine at the European level. EU-LIFE introduced an overview of recommendations that should improve the situation during the new seven-year Horizon Europe research and innovation programme and have a long-term impact.
EU-LIFE, the alliance of 13 leading life science institutes in Europe, points out the increasing lack of opportunities for collaborative research in biomedicine at the European level. EU-LIFE introduced an overview of recommendations that should improve the situation during the new seven-year Horizon Europe research and innovation programme and have a long-term impact.
As a member of EU-LIFE, CEITEC Masaryk University (MU), the research institute located in Brno, Czech Republic, is joining this initiative. “It is necessary to realize that the European grant system to support research has a certain logic and strategy,“ explains the complexity of the problem Jiří Nantl, Director of CEITEC MU. “ERC elite schemes have a crucial role in supporting and profiling the discovery research, where increase in allocation leads to increase in chances of success. However, we also need robust funding for inter-institutional collaborative projects to achieve ambitious broader research plans than on the level of individual team project.”
EU-LIFE calls for impactful collaborative research in European biomedicine: 6 recommendations for the Strategic programming of Horizon Europe’s Health Cluster
EU-LIFE, the alliance of 13 leading life science institutes in Europe, points out the increasing lack of opportunities for collaborative research in biomedicine at a European level and highlights how this is endangering long-lasting, positive impact in health research for the benefit of citizens. Based on the analysis on the barriers regarding participation in collaborative health research in Horizon 2020, 6 recommendations aim at contributing to improvement for the upcoming seven-year cycle of Horizon Europe.
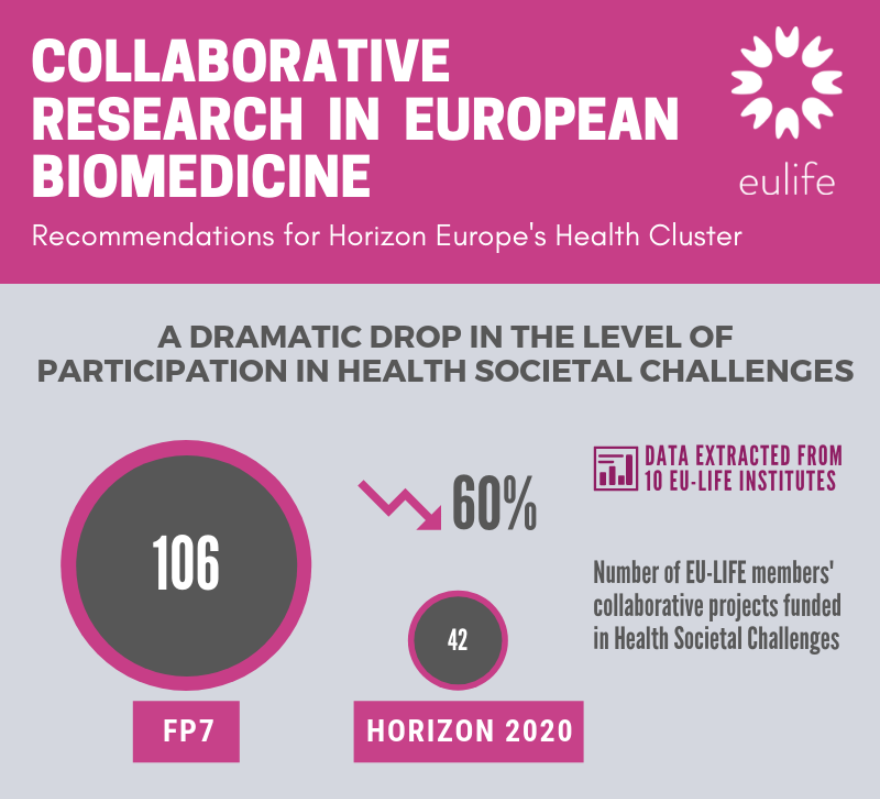
A dramatic drop in the level of participation in Health Societal Challenges in H2020
EU-LIFE institutes have observed that there were limited opportunities for collaborative research in biomedicine in Horizon 2020. Data extracted from 10 EU-LIFE member institutes confirms this observation. It demonstrates a 60% drop in the level of participation of these organisations in Health Societal Challenges (SC1) consortia between FP7, the European Research & Innovation programme running from 2007-2013, and Horizon 2020, the current EU programme (107 collaborative projects in FP7 compared to only 42 in Horizon 2020). This dramatic drop in the level of participation within Horizon 2020 can partly be explained by limited number of calls focussed on understanding mechanisms and fundamental principles in health and disease (reflecting a low Technology Readiness Level - TRL). For example, in the Health 2014-2015 Work Programme on “Personalising health and care”, only 3 calls focussed on understanding mechanisms of health and disease while 31 focussed on developing diagnosis, ICT, innovative technologies, care systems and health policy.
Long-term impact on health care only sustainable with a flow of knowledge from discovery research
While it is important to ensure further translation of discovery research to create impact in peoples’ lives, increasing consensus shows that real health impact is not sustainable without strong and continued support for research aimed at understanding the fundamental mechanisms behind health and disease. Many questions about health and disease, operating in different global environments, need an urgent answer. Long-lasting innovation requires international research collaborations and without it, we risk losing the scientific foundations of future impact.
6 recommendations for collaborative research in the Health Cluster of Horizon Europe
- Re-balance public funding in the Health Cluster towards collaborative, long-term impact research and leave the investment in short-term research (at higher TRLs) to the private sector. Whereas the less risky, close-to-market stages are amenable to private funding, lower TRLs that encapsulate longer-term impact are higher risk and therefore not fundable by industry or the private sector - this should be the main responsibility of public funding in Horizon Europe.
- Define major challenges for specific disease areas and include calls for proposals on “understanding mechanisms of” to ensure collaborative approaches regarding the fundamental understanding of mechanisms that form the knowledge base of disease and treatment.
- Build a more realistic definition of impact. Impact in the current calls requires heavy speculation as to what might happen often ending in hand waiving and the description of “impact unicorns”. EU-LIFE recommends a shift towards explaining how the current research environment facilitates further development and exploitation to enable research impact.
- Use a wider and modular definition of “expected impacts” by taking into account what is more commonly the result of discovery research in the area of the call, and by including “fundamental understandings” as expected impact. Value the collaborative aspect of the project as a measure of impact. Brief officers and evaluators to appreciate the impact of research for the area and TRL in question.
- Remove the - direct or indirect - pressure to cover too many and too high TRLs in a single project by introducing several stages for a research theme: the first stage starting at lower TRLs and if successful progressing to higher TRLs.
- Implement a retrospective model of evaluation for collaborative research: Rather than evaluating “unicorns”, evaluate a track record of generating impact. Look back at what research has achieved following funding at the portfolio or programme level.
“Great discoveries of the past centuries tell us that a short-term vision of impact does not contribute to long-standing, sustainable impact”, says Geneviève Almouzni, Chair of EU-LIFE and Head of the Chromatin Dynamics team at Institut Curie, France. “Addressing unmet medical needs requires a return to a more balanced portfolio of research across the entire research and innovation spectrum. This is the only way to ensure a steady flow of powerful innovations from the bench to bedside.”
“The path from discovery to innovation is not linear and includes many feedback loops. Long-lasting innovation requires international research collaborations and without it, we risk losing the scientific foundations of future impact,” says Marta Agostinho, EU-LIFE Coordinator.
About EU-LIFE
EU-LIFE is an alliance of research centres whose mission is to support and strengthen European research excellence (www.eu-life.eu). EU-LIFE members are leading research institutes in their countries and internationally renowned for producing excellent research, widely transferring knowledge and nurturing talent.
EU-LIFE Partners
Center for Genomic Regulation (CRG, Spain) | Central European Institute of Technology (CEITEC, Czech Republic) | European Institute of Oncology (IEO, Italy) | Flanders Institute For Biotechnology (VIB, Belgium) | Friedrich Miescher Institute for Biomedical Research (FMI, Switzerland) | Institut Curie (France) | Institute for Molecular Medicine Finland (FIMM, Finland) | Instituto Gulbenkian de Ciência (IGC, Portugal) | Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC, Germany) | Research Center for Molecular Medicine of the Austrian Academy of Sciences (CeMM, Austria) | The Babraham Institute (Babraham, United Kingdom) | The Netherlands Cancer Institute (NKI, The Netherlands) | The University of Copenhagen Biotech Research & Innovation Centre (BRIC, Denmark)
For more information
Marta Agostinho, PhD
EU-LIFE Coordinator
Email: marta.agostinho@eu-life.eu
Mobile: +34619570820
Ana-Belén Fernandez
EU-LIFE Communications officer
Email: anabelen.fernandez@eu-life.eu
Phone: +34933160379


 Share
Share